A New Frontier in Breast Cancer Treatment
presented by: AstraZeneca
1. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer
![]()
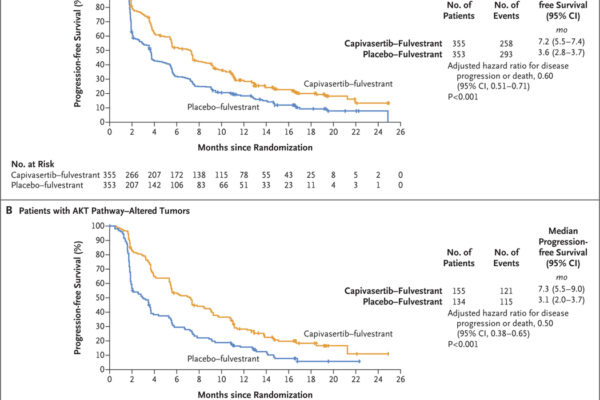
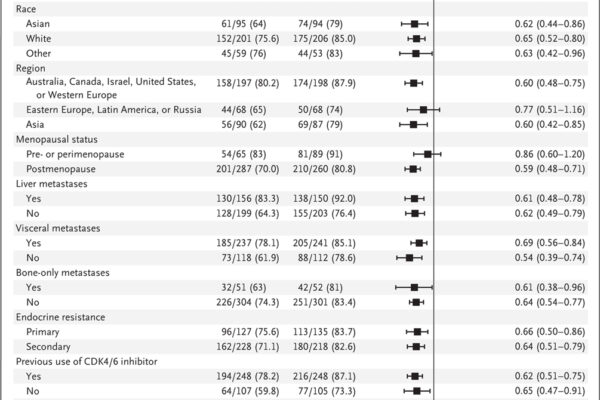
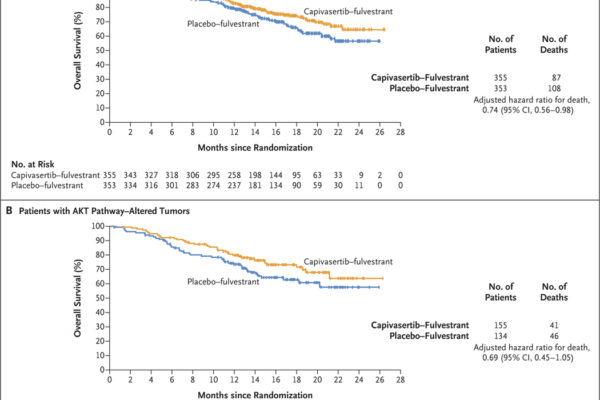
A phase 3 trial assessed capivasertib combined with fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. The combination significantly improved progression-free survival compared to placebo plus fulvestrant, with notable benefits in patients harboring AKT pathway alterations.
2. Capivasertib Plus Paclitaxel as First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial
![]()
This randomized phase 2 trial evaluated capivasertib with paclitaxel versus placebo with paclitaxel in untreated metastatic triple-negative breast cancer. The addition of capivasertib led to improved progression-free and overall survival, especially in patients with PI3K/AKT pathway alterations.
3. The Emerging Role of Capivasertib in Breast Cancer

A comprehensive review highlighted capivasertib’s potential across breast cancer subtypes, emphasizing its efficacy in tumors with aberrant PI3K/AKT signaling. Ongoing trials are further exploring its role in combination therapies.
4. Effect of Capivasertib in Patients With an AKT1 E17K-Mutated Tumor: NCI-MATCH Subprotocol EAY131-Y Nonrandomized Trial

This nonrandomized trial focused on capivasertib’s activity in patients with tumors harboring the AKT1 E17K mutation. The study reported a modest overall response rate, suggesting limited single-agent activity in heavily pretreated populations.

Find out if TRUQAP could be right for your patients.
TRUQAP is for people with HR+/HER2- metastatic breast cancer who test positive for abnormal PIK3CA, AKT1, and/or PTEN genes
Take Control NowCapivasertib: Advancing Breast Cancer Treatment
Capivasertib, an oral AKT inhibitor, has demonstrated significant efficacy in various breast cancer subtypes, particularly those with PI3K/AKT pathway alterations. Clinical trials have shown that combining capivasertib with standard therapies enhances progression-free and overall survival in patients with hormone receptor-positive advanced breast cancer and metastatic triple-negative breast cancer. Ongoing research continues to define its role in targeted cancer therapy, offering hope for more effective and personalized treatment options.

AstraZeneca in the United States
We are a global, science-led biopharmaceutical business and our innovative medicines are used by millions of patients worldwide.
A Targeted Approach for Advanced Breast Cancer
Capivasertib offers healthcare providers a powerful option in the management of advanced breast cancer, specifically hormone receptor-positive and triple-negative subtypes. By targeting the PI3K/AKT signaling pathway, this therapy provides a precision-based approach to improving progression-free survival and overall outcomes in patients with challenging disease profiles. Backed by robust clinical data, Capivasertib demonstrates significant efficacy in combination regimens, empowering you to deliver cutting-edge care and optimize patient outcomes in the evolving landscape of oncology.